- Model Number: TREO24
- Product Description This product consists of Bacillus atrophaeus (ATCC9372) spores and culture medium. Post-incubation color change confirms spore viability, thereby validating the efficacy of ethylene oxide (EO) sterilization. Spore Load: 1×106 - 1×107 cfu/unit.
- Intended Use For monitoring the efficacy of EO sterilization processes.
EO Biological Indicator 24 Hours
Description
Operating Instructions
1.Blank space of label on surface of indicator, record necessary matters for sterilization management (such as: sterilization processing date, operator, etc.)
2.Put the indicator into a standard packing bag, then place it in the most difficult to sterilize position in the sterilizer and run sterilization procedures.
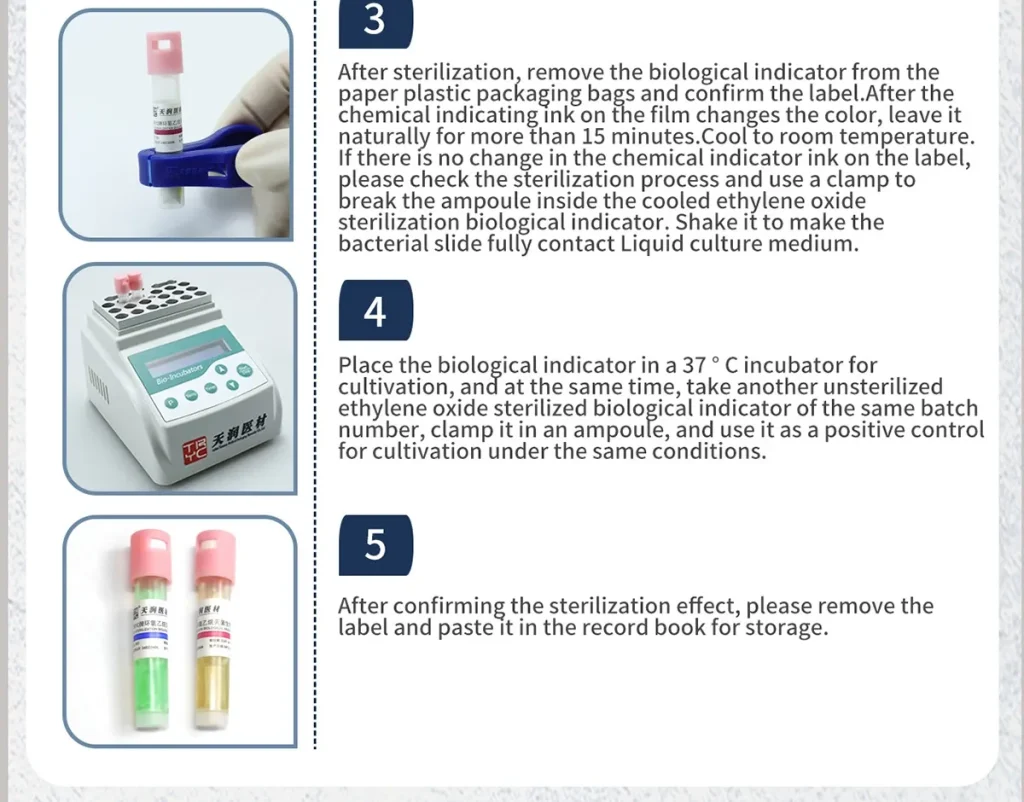
3.After sterilization is completed, take out the indicator from the paper-plastic bag. After confirming that the chemical indicator ink on the label has changed color, leave it naturally to cool to room temperature. If there is no change in the chemical indicator ink on the label, check the sterilization process.
4.Use a clamp to break the ampoule inside the cooled indicator, shake it to make the bacterial pieces fully contact the liquid culture medium,then culture it in an incubator at 37 ° C.
5.Take another unsterilized indicator of the same batch number, and after clipping the ampoule, culture it under the same conditions as a positive control.
6.After confirming the sterilization effect, please remove the label and paste it in the record book for storage.
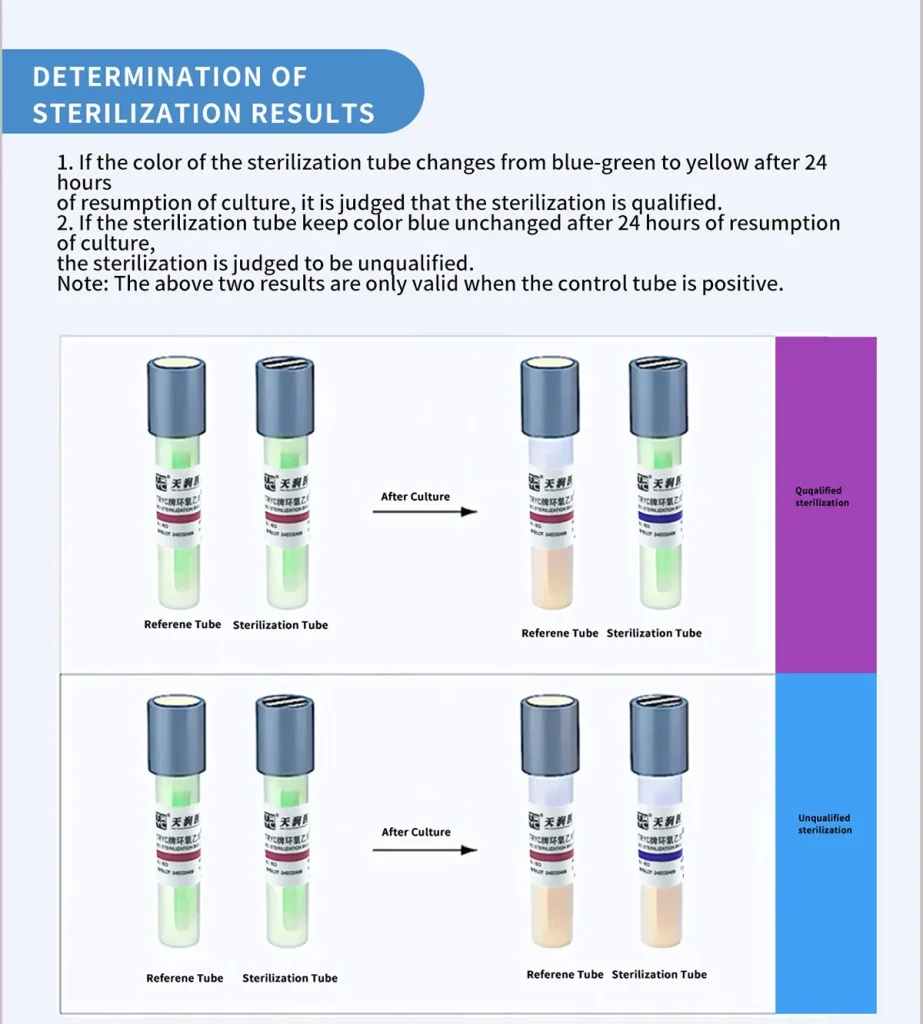
Result judgment:
1.After 24 h,culture medium color green to yellow, unqualified sterilization.
2.After 24 h,culture medium keep green color unchanged, qualified sterilization.
3.Test results are valid only if the reference tube is positive.
7. Precautions
1.Before using product, please confirm integrity of product and use it within validity period.
2.Store in a dry environment at 15℃-25℃ (relative humidity 35%-60%), away from light 3.Indicators that are judged to have failed sterilization, have expired, or are used in positive control tests should be discarded after sterilization.
4.This product is only used for biological monitoring of EO sterilization process.
8.Validity period: 24 months. production date, batch number, specifications be found product packaging.