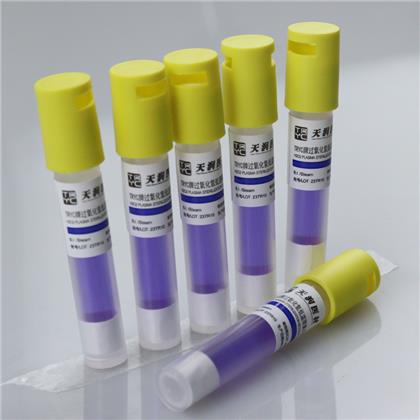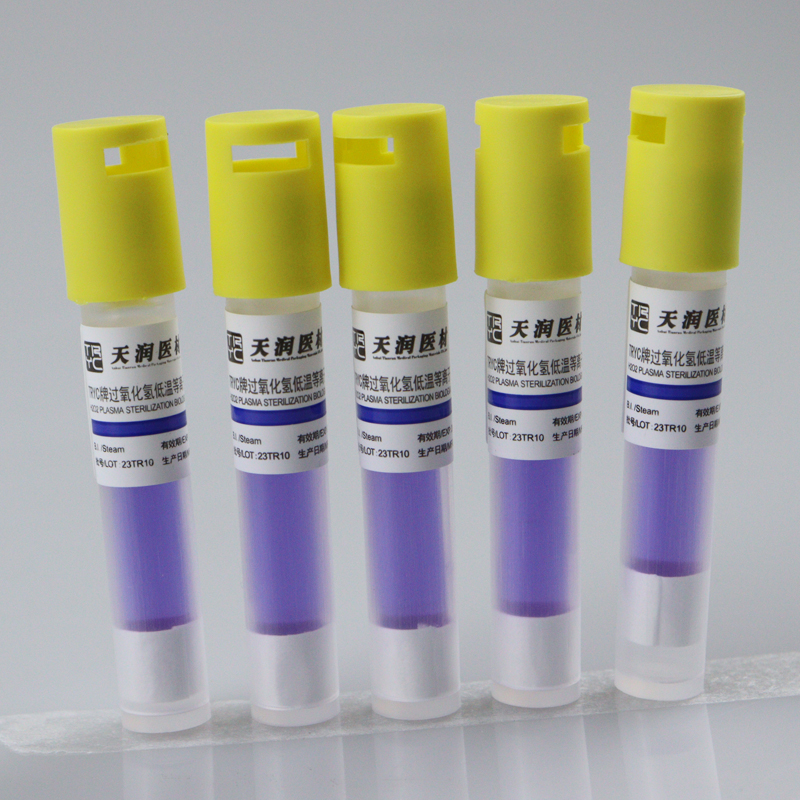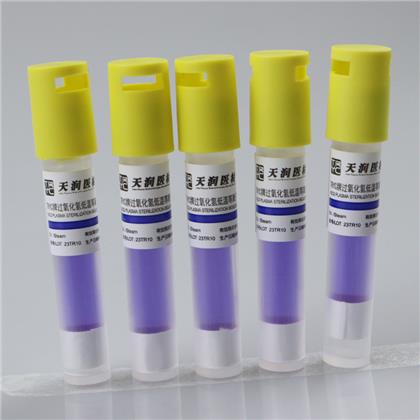
Hydrogen peroxide low temperature plasma biological indicator
TRYC brand hydrogen peroxide low temperature plasma sterilization biological indicator instruction manualI. Product Description
This product is composed of Bacillus stearothermophilus (ATCC7953) and bromocresol purple peptone culture medium. According to the color change after culture, whether hydrogen peroxide low-temperature plasma sterilization is qualified or not can be judged. The spore content of this product is above 1×106cfu/ branch.
Second, the scope of use
It is suitable for monitoring the sterilization effect of hydrogen peroxide low temperature plasma.
Third, the method of use
1. Record the necessary items of sterilization management (such as sterilization date, operator, etc.) in the blank space of the label on the surface of the biological indicator sterilized by hydrogen peroxide at low temperature plasma.
2. Put the biological indicator sterilized by hydrogen peroxide low temperature plasma into a Tyvek packaging bag, and then put it in the position that is difficult to sterilize in the sterilizer recommended by the manufacturer (generally in the lower front of the equipment), and run the sterilization procedure.
3. After sterilization, take out the biological indicator from the paper-plastic bag, and after confirming that the chemical indicator ink on the label changes color, naturally leave it for more than 15min and cool it to room temperature. If the chemical indication ink on the label has not changed, please check the sterilization process.
4. Press the upper cover of the biological indicator tightly, use a clamp to break the ampoule bottle inside the cooled pressure steam sterilization biological indicator, shake it to make the bacterial slices fully contact with the liquid culture medium, and culture it in time after confirming that the bacterial slices are immersed in the culture medium.
5. Please cultivate at 56 ~ 58℃. Another unsterilized biological indicator of hydrogen peroxide low-temperature plasma sterilization with the same batch number was taken, and after the ampoule bottle was broken, it was cultured as a positive control under the same conditions.
6. After confirming the sterilization effect, please take off the label and stick it in the record book.
Result judgment:
1. If the culture medium changes from purple to yellow after 24 hours, it means that the sterilization is unqualified.
2. If the color of the culture medium remains purple after 24 hours of culture, it means that the sterilization is qualified.
3. The above two results are valid only when the control tube is positive.
Fourth, matters needing attention
1. Before using this product, please confirm the integrity of the product and use it within the validity period.
2. Store at 4℃-25℃ in a dry environment (relative humidity 35%-60%) and avoid light (including sunlight, fluorescent lamps and ultraviolet disinfection lights).
3. Biological indicators that have been judged to have failed sterilization, expired and used in positive control tests should be discarded after sterilization.
4. This product is only used for biological monitoring of hydrogen peroxide low-temperature plasma sterilization process.
5. Validity: 24 months. See the product packaging for the production date, batch number and specifications.
Manufacturer: Anhui Tianrun Medical New Materials Co., Ltd.
Production address: No.29 Chaoyang Road, Yixiu District, Anqing City, Anhui Province
Production license: Wan Wei Xiao Zheng Zi [2023]No. H0047
Tel: 0556-5877077 Postal code: 246500
Fax: 0556-5877079 Website: www.ahtrbz.com.
1. Record the necessary items of sterilization management (such as sterilization date, operator, etc.) in the blank space of the label on the surface of the biological indicator sterilized by hydrogen peroxide at low temperature plasma.
2. Put the biological indicator sterilized by hydrogen peroxide low temperature plasma into a Tyvek packaging bag, and then put it in the position that is difficult to sterilize in the sterilizer recommended by the manufacturer (generally in the lower front of the equipment), and run the sterilization procedure.
3. After sterilization, take out the biological indicator from the paper-plastic bag, and after confirming that the chemical indicator ink on the label changes color, naturally leave it for more than 15min and cool it to room temperature. If the chemical indication ink on the label has not changed, please check the sterilization process.
4. Press the upper cover of the biological indicator tightly, use a clamp to break the ampoule bottle inside the cooled pressure steam sterilization biological indicator, shake it to make the bacterial slices fully contact with the liquid culture medium, and culture it in time after confirming that the bacterial slices are immersed in the culture medium.
5. Please cultivate at 56 ~ 58℃. Another unsterilized biological indicator of hydrogen peroxide low-temperature plasma sterilization with the same batch number was taken, and after the ampoule bottle was broken, it was cultured as a positive control under the same conditions.
6. After confirming the sterilization effect, please take off the label and stick it in the record book.
Result judgment:
1. If the culture medium changes from purple to yellow after 24 hours, it means that the sterilization is unqualified.
2. If the color of the culture medium remains purple after 24 hours of culture, it means that the sterilization is qualified.
3. The above two results are valid only when the control tube is positive.
Fourth, matters needing attention
1. Before using this product, please confirm the integrity of the product and use it within the validity period.
2. Store at 4℃-25℃ in a dry environment (relative humidity 35%-60%) and avoid light (including sunlight, fluorescent lamps and ultraviolet disinfection lights).
3. Biological indicators that have been judged to have failed sterilization, expired and used in positive control tests should be discarded after sterilization.
4. This product is only used for biological monitoring of hydrogen peroxide low-temperature plasma sterilization process.
5. Validity: 24 months. See the product packaging for the production date, batch number and specifications.
Manufacturer: Anhui Tianrun Medical New Materials Co., Ltd.
Production address: No.29 Chaoyang Road, Yixiu District, Anqing City, Anhui Province
Production license: Wan Wei Xiao Zheng Zi [2023]No. H0047
Tel: 0556-5877077 Postal code: 246500
Fax: 0556-5877079 Website: www.ahtrbz.com.





- Previous:Pressure steam sterilization speed biological indicator
- Next:Pressure steam biological indicator
Online consulting