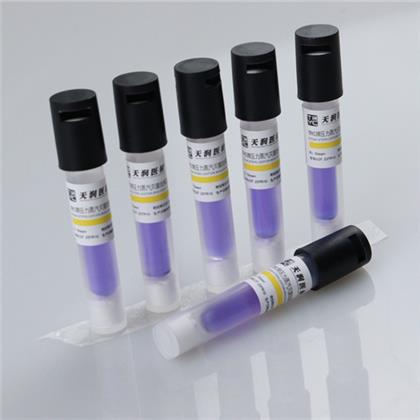
Pressure steam biological indicator
TRYC brand pressure steam sterilization biological indicator instruction manualI. Product Description
This product is composed of Bacillus stearothermophilus (ATCC7953) and bromocresol purple peptone recovery medium. According to the color change after culture, whether pressure steam sterilization is qualified or not can be judged. The spore content of this product is 5×105cfu/ branch -5×106cfu/ branch, and the death time and survival time of pressure steam sterilization at 121℃ are not more than 19 minutes and 3.9 minutes respectively.
Second, the scope of use
It is suitable for monitoring the sterilization effect of pressure steam.
Third, the method of use
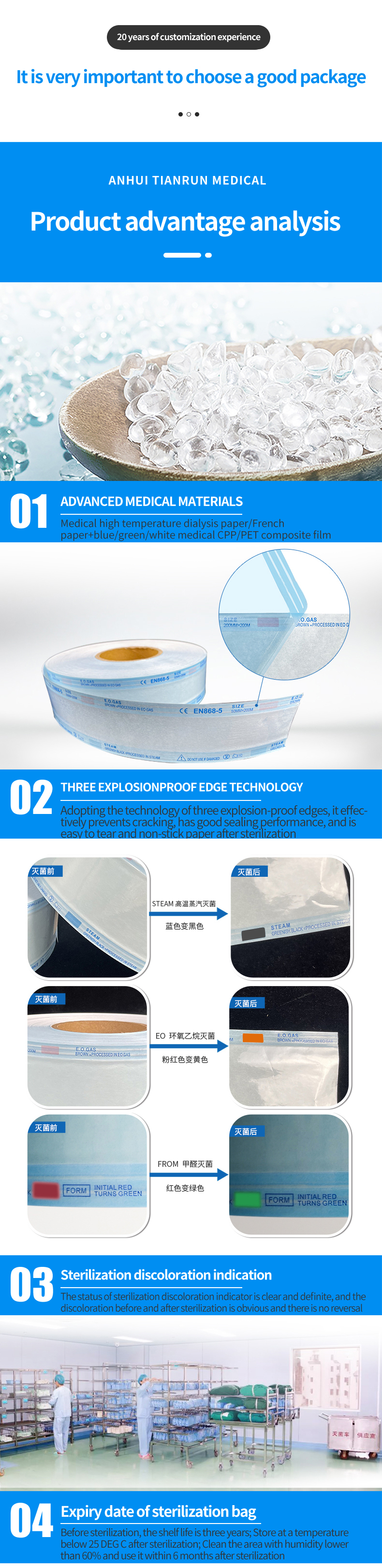
1. Record the necessary items of sterilization management (such as sterilization date, operator, etc.) in the blank space of the label on the surface of the biological indicator for pressure steam sterilization.
2. Place the biological indicator for pressure steam sterilization in the center of the standard test package, and then place the standard test package above the exhaust port of the sterilizer or at the position that is difficult to sterilize in the sterilizer suggested by the manufacturer, and run the sterilization procedure.
3. After sterilization, take out the biological indicator in the standard test package, and after confirming that the chemical indicator ink on the label changes color, naturally leave it for more than 15min and cool it to room temperature. If the chemical indication ink on the label has not changed, please check the sterilization process.
4. Clamp the ampoule bottle inside the cooled pressure steam sterilization biological indicator with a clamp, and shake it to make the bacterial slices fully contact with the liquid culture medium, and culture it in time after confirming that the bacterial slices are immersed in the culture medium.
5. Please cultivate at 56 ~ 58℃. Another unsterilized biological indicator with the same batch number was taken, and after the ampoule was broken, it was cultured as a positive control under the same conditions.
6. After confirming the sterilization effect, please take off the label and stick it in the record book.
Result judgment:
1. If the culture medium changes from purple to yellow after 24 hours, it means that the sterilization is unqualified.
2. If the color of the culture medium remains purple after 24 hours of culture, it means that the sterilization is qualified.
3. The above two results are valid only when the control tube is positive.
Fourth, matters needing attention
1. Before using this product, please confirm the integrity of the product and use it within the validity period.
2, stored at 15℃-25℃ and dry environment (relative humidity 35%-60%), away from light (including sunlight, fluorescent lamps and ultraviolet disinfection lights).
3. Biological indicators that are judged to have failed sterilization, expired and used in positive control tests should be discarded after sterilization.
4. This product cannot be used for biological monitoring of dry heat, ethylene oxide and other low-temperature sterilization processes.
5. Validity: 24 months. See the product packaging for the production date, batch number and specifications.
Manufacturer: Anhui Tianrun Medical New Materials Co., Ltd.
Production address: No.29 Chaoyang Road, Yixiu District, Anqing City, Anhui Province
Production license: Wan Wei Xiao Zheng Zi [2023]No. H0047
Tel: 0556-5877077 Postal code: 246500
Fax: 0556-5877079 Website: www.ahtrbz.com.
1. Record the necessary items of sterilization management (such as sterilization date, operator, etc.) in the blank space of the label on the surface of the biological indicator for pressure steam sterilization.
2. Place the biological indicator for pressure steam sterilization in the center of the standard test package, and then place the standard test package above the exhaust port of the sterilizer or at the position that is difficult to sterilize in the sterilizer suggested by the manufacturer, and run the sterilization procedure.
3. After sterilization, take out the biological indicator in the standard test package, and after confirming that the chemical indicator ink on the label changes color, naturally leave it for more than 15min and cool it to room temperature. If the chemical indication ink on the label has not changed, please check the sterilization process.
4. Clamp the ampoule bottle inside the cooled pressure steam sterilization biological indicator with a clamp, and shake it to make the bacterial slices fully contact with the liquid culture medium, and culture it in time after confirming that the bacterial slices are immersed in the culture medium.
5. Please cultivate at 56 ~ 58℃. Another unsterilized biological indicator with the same batch number was taken, and after the ampoule was broken, it was cultured as a positive control under the same conditions.
6. After confirming the sterilization effect, please take off the label and stick it in the record book.
Result judgment:
1. If the culture medium changes from purple to yellow after 24 hours, it means that the sterilization is unqualified.
2. If the color of the culture medium remains purple after 24 hours of culture, it means that the sterilization is qualified.
3. The above two results are valid only when the control tube is positive.
Fourth, matters needing attention
1. Before using this product, please confirm the integrity of the product and use it within the validity period.
2, stored at 15℃-25℃ and dry environment (relative humidity 35%-60%), away from light (including sunlight, fluorescent lamps and ultraviolet disinfection lights).
3. Biological indicators that are judged to have failed sterilization, expired and used in positive control tests should be discarded after sterilization.
4. This product cannot be used for biological monitoring of dry heat, ethylene oxide and other low-temperature sterilization processes.
5. Validity: 24 months. See the product packaging for the production date, batch number and specifications.
Manufacturer: Anhui Tianrun Medical New Materials Co., Ltd.
Production address: No.29 Chaoyang Road, Yixiu District, Anqing City, Anhui Province
Production license: Wan Wei Xiao Zheng Zi [2023]No. H0047
Tel: 0556-5877077 Postal code: 246500
Fax: 0556-5877079 Website: www.ahtrbz.com.





Online consulting